Polypropylene (PP) is a widely used thermoplastic polymer known for its versatility and durability. This lightweight yet strong material plays a crucial role in various industries, ranging from packaging and automotive manufacturing to healthcare and textiles. Due to its excellent chemical resistance, cost-effectiveness, and recyclability, polypropylene has become a preferred choice for countless applications. In this article, we will explore the diverse Polypropylene uses and properties, highlighting its key properties and benefits that make it indispensable in modern industries.
What is Polypropylene (PP)?
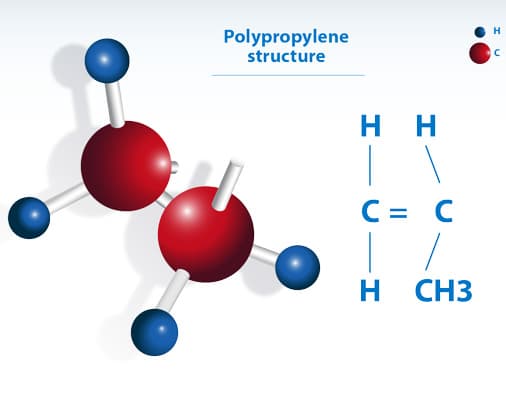
Polypropylene (PP) is a widely used thermoplastic polymer composed of repeating units of propylene (C₃H₆). Its molecular structure consists of a linear hydrocarbon backbone with hydrogen and methyl (-CH₃) groups attached to every other carbon atom. This unique polypropylene chemical structure enhances its mechanical strength, flexibility, and resistance to moisture and chemicals, making it highly durable and suitable for various applications.
The polypropylene chemical formula is (C₃H₆)ₙ, indicating that it is made up of multiple linked monomers. This structure plays a key role in defining the polypropylene pp structure, influencing its crystallinity, rigidity, and thermal stability. polypropylene structure can exist in different forms, including isotactic, syndiotactic, and atactic polypropylene, each with distinct properties that affect its performance in different applications.
Due to its versatile nature, polypropylene is used across various industries. Some pp examples include storage containers, automotive components, medical syringes, and industrial piping. Additionally, polypropylene examples extend to food packaging, ropes, laboratory equipment, and outdoor furniture. Its lightweight nature, durability, and recyclability make it a preferred choice in packaging, textiles, construction materials, and medical devices. PP’s resistance to heat, chemicals, and mechanical stress further solidifies its importance in both consumer and industrial products.

Polypropylene pp structure
- Monomer Structure: Propylene (C₃H₆) has the structure CH₂=CHCH₃, with a double bond between the first and second carbon atoms.
- Polymerization: During polymerization (typically using Ziegler-Natta catalysts), the double bond in propylene opens, allowing monomers to link in a head-to-tail fashion. This forms a linear chain with repeating units of -CH₂-CH(CH₃)-.
- Repeating Unit: The repeating unit is -[CH₂-CH(CH₃)]ₙ-, where n denotes the number of repeating units. The backbone consists of carbon atoms with a methyl (CH₃) group attached to every other carbon.
4. Tacticity: The spatial arrangement of methyl groups determines PP’s tacticity:
-
- Isotactic: All methyl groups are on the same side of the chain, creating a helical structure. This arrangement is semi-crystalline, offering high strength and a melting point (~160–170°C). Most commercial PP is isotactic.
- Syndiotactic: Methyl groups alternate sides, leading to lower crystallinity.
- Atactic: Random methyl group placement results in an amorphous, rubbery material (less commercially useful).
5. Molecular Formula: The polymer’s formula is (C₃H₆)ₙ, with a molecular weight varying by chain length.
6. 3D Structure: Isotactic PP forms a helical conformation, enabling tight packing and crystallinity (30–60%), which enhances mechanical properties and thermal resistance.
History of Polypropylene
polypropylene pp was first polymerized in 1951 by Paul Hogan and Robert Banks at Phillips Petroleum. However, its commercial potential was realized in 1954 when Giulio Natta and Karl Ziegler developed a stereoregular form of PP using a Ziegler-Natta catalyst. This breakthrough led to large-scale production, and by the 1960s, polypropylene became a widely used thermoplastic due to its versatility, durability, and cost-effectiveness. Today, it remains one of the most important polymers in various industries worldwide.
Types of Polypropylenes
Polypropylene exists in various forms, each designed to meet specific industrial and commercial needs. The variations in structure and composition result in differences in mechanical strength, flexibility, impact resistance, and processability. Examples of polypropylene are presented below. pp examples include:
This is the most common type of PP, consisting of only propylene monomers. It offers high rigidity, good chemical resistance, and thermal stability, making it ideal for packaging, textiles, and automotive applications.
Copolymers are modified versions of homopolymer PP, incorporating ethylene to enhance impact resistance and flexibility. They come in two main types:
- Random Copolymer: Contains small amounts of ethylene, improving clarity and flexibility. Used in transparent packaging and medical devices.
- Block Copolymer: Has higher ethylene content, forming tougher polymer blocks. It provides superior impact resistance, suitable for automotive and industrial applications.
- Polypropylene Impact Copolymer
This type of copolymer has ethylene monomers randomly distributed within the polymer. It offers a balance between stiffness and impact resistance, making it suitable for applications like food containers, closures, and other consumer goods
- Block Copolymer Polypropylene
This type contains a higher ethene content (between 5 and 15%) and has co-monomer units arranged in a regular pattern (or blocks). These polymers are suitable for applications requiring high strength, such as industrial usages
- Impact Copolymer Polypropylene
A specialized block copolymer with an additional rubber phase, offering exceptional toughness and durability. Commonly used in bumpers, storage containers, and industrial components.
- Expanded Polypropylene (EPP)
A lightweight foam variant of PP with superior shock absorption, thermal insulation, and recyclability. Used in automotive safety components, packaging, and sports equipment.
- Polypropylene Terpolymer
A blend of propylene, ethylene, and butene, offering enhanced flexibility, transparency, and heat resistance. It is mainly used in high-performance films, adhesives, and medical applications.
- High Melt Strength Polypropylene (HMS-PP)
This type features long-chain branching, improving melt strength and elasticity. It is ideal for thermoforming, blow molding, and foaming applications in packaging and automotive industries.
- Bio-based Polypropylene
An eco-friendly alternative produced from renewable sources, such as sugarcane or corn-based bioethanol. It retains the properties of conventional PP while reducing carbon footprint, making it an attractive option for sustainable packaging and green manufacturing.
Understanding Polypropylene Properties
Polypropylene (PP) is valued for its exceptional balance of mechanical, thermal, and chemical properties, making it a preferred material in various industries. Below is a detailed overview of its key characteristics.
Mechanical Properties
- Tensile Strength: PP offers moderate tensile strength, making it suitable for applications requiring flexibility and durability.
- Rigidity: Homopolymer PP is highly rigid, while copolymers offer improved impact resistance.
- Impact Resistance: Although PP is generally tough, it can become brittle at very low temperatures.
Thermal Properties
- Melting Point: As a polypropylene thermoplastic, PP has a melting point ranging from 130°C to 170°C, depending on whether it is a homopolymer or copolymer. It also exhibits excellent heat resistance and a low coefficient of thermal expansion, making it suitable for high-temperature applications.
- Heat Resistance: PP exhibits excellent resistance to heat, with a low coefficient of thermal expansion, making it ideal for applications exposed to high temperatures.
Chemical Properties
- Resistance to Acids, Solvents, and Bases: One of the key Polypropylene properties is its excellent chemical resistance, making it suitable for industrial and medical applications. PP remains stable when exposed to dilute acids, alkalis, and organic solvents, making it an ideal choice for chemical storage, piping, and laboratory equipment.
- Degradation by Strong Oxidizers: Prolonged exposure to strong oxidizers (e.g., sulfuric acid, nitric acid, and peroxides) can cause polymer breakdown.
- Effect of Aromatic Hydrocarbons: Contact with benzene, toluene, or xylene may lead to swelling or softening, affecting mechanical properties.
Density
One of the key polypropylene properties is its low density, making it lighter than many other thermoplastics. The polypropylene density typically ranges between 0.89–0.91 g/cm³, which is lower than materials like polyethylene (PE) and polyvinyl chloride (PVC).
- Comparison with Other Plastics: The polypropylene density is significantly lower than PVC (~1.38 g/cm³) or PET (~1.37 g/cm³), making it an excellent choice for lightweight applications.
- Advantages of Low Density: Due to its low weight and high strength, PP is widely used in automotive parts, packaging materials, and consumer goods, where material efficiency is essential.
- Industrial Applications: The polypropylene density contributes to its extensive use in textiles, food containers, and medical devices, offering a balance between durability and lightweight performance.
Molecular Structure: Tacticity
The tacticity of polypropylene refers to the arrangement of methyl (-CH₃) groups along the polypropylene polymer chain, directly influencing crystallinity, strength, and flexibility. Depending on this arrangement, polypropylene can exist in three distinct forms, each with unique characteristics.
Crystal Structure of Polypropylene
- Isotactic Polypropylene (iPP): A highly ordered structure where all methyl groups align on the same side of the polymer backbone. This results in high crystallinity, excellent rigidity, and superior mechanical strength, making it ideal for pipes, packaging, and automotive parts.
- Syndiotactic Polypropylene (sPP): Features an alternating arrangement of methyl groups, leading to moderate crystallinity and enhanced flexibility. This variant offers better impact resistance and is commonly used in films and coatings.
- Atactic Polypropylene (APP): Lacks a defined pattern in the placement of methyl groups, making it amorphous and rubber-like. It is primarily used in adhesives and sealants due to its soft and tacky nature.
Copolymers
One of the notable features of polypropylene is its ability to exist as a copolymer, which enhances its physical and mechanical properties for various applications.
PP-RCT (Polypropylene Random Crystallinity Temperature)
PP-RCT is an advanced type of random copolymer polypropylene with enhanced crystallinity control. It offers higher pressure resistance at elevated temperatures, making it particularly effective in hot water piping systems and industrial fluid transport.
Degradation
Polypropylene is highly resistant to environmental factors, but certain conditions can lead to degradation:
UV Exposure: Prolonged sunlight exposure can cause photo-oxidation, leading to brittleness and discoloration. UV stabilizers are often added to prevent this.
Thermal Degradation: Excessive heat can break down the polymer chains, reducing mechanical strength.
Chemical Degradation: Strong oxidizers, acids, and aromatic hydrocarbons can cause molecular breakdown, impacting performance.
Optical properties
Polypropylene’s optical clarity varies depending on its crystallinity and processing conditions:
- Homopolymer PP: Has a higher crystalline structure, making it opaque.
- Random Copolymer PP: Features lower crystallinity, enhancing transparency and making it suitable for clear packaging and medical applications.
- Effect of Additives: Clarifiers and nucleating agents can improve light transmission and surface gloss, enhancing the aesthetic quality of products.
How Polypropylene is Made

Polypropylene is produced through the polymerization of propylene monomers, using specialized catalysts to control molecular weight, tacticity, and crystallinity.
- Ziegler-Natta Polymerization: The most common method, using Ziegler-Natta catalysts to produce isotactic polypropylene with high crystallinity.
- Metallocene Catalysis Polymerization: A newer technique that enables precise control over polymer structure, producing high-performance PP with enhanced properties.
- Catalysts: Different catalysts influence molecular arrangement, affecting the mechanical strength, flexibility, and transparency of Polypropylene products.
Manufacturing and Processing Polypropylene
Polypropylene can be processed using various methods, depending on the final product requirements:
- CNC Machining: Used for precision parts and prototypes.
- Injection Molding: Ideal for mass production of complex shapes, such as automotive and medical components.
- Extrusion: Produces pipes, sheets, and films by forcing molten PP through a shaped die.
- 3D Printing: Enables custom PP parts, though it requires specialized printers due to PP’s low adhesion.
- Biaxially Oriented Polypropylene (BOPP): A process that stretches PP film in two directions, enhancing its clarity, strength, and barrier properties for packaging applications.
Polypropylene Products and Forms
Polypropylene products come in various forms, serving industries like packaging, automotive, and construction.
- Polypropylene Sheet: Lightweight, durable, and chemical-resistant, used in automotive parts, medical trays, and signage.
- Injection-Molded Parts: Found in automotive interiors, containers, and medical equipment, benefiting from PP’s heat resistance.
- Fibers and Textiles: Used in ropes, carpets, and nonwoven fabrics, offering moisture and chemical resistance.
- Films (BOPP, CPP):
- BOPP: High-clarity, tear-resistant film for food packaging and labeling.
- CPP: More flexible, used in medical and heat-sealable packaging.
Applications of Polypropylene: Industry Focus
Polypropylene uses in industry cover multiple sectors due to its durability, chemical resistance, and cost-effectiveness.
- Packaging Industry: Used in food and industrial packaging for its moisture resistance and lightweight nature.
- Textiles: Applied in carpets, ropes, and fabrics, benefiting from its strength and moisture resistance.
- Household & Consumer Goods: Found in appliances, furniture, and personal care items for its durability.



- Automotive Industry: Found in bumpers, dashboards, and interior trims, offering impact strength and heat resistance.
- Medical Field: Used in syringes, medical devices, and sterile packaging due to its biocompatibility.


Polypropylene Uses in Agriculture
Polypropylene uses in agriculture include:
- Geotextiles: Used for soil stabilization and erosion control.
- Crop Protection Fabrics: Shield crops from extreme weather conditions.
- Ropes and Twines: Strong, lightweight, and weather-resistant.
- Agricultural Films: Enhance soil moisture retention and weed control.

Advantages of Using Polypropylene
-
Chemical Resistance
Polypropylene exhibits exceptional resistance to a wide range of chemicals, including strong acids (e.g., sulfuric, hydrochloric), alkaline bases (e.g., sodium hydroxide), and organic solvents. This makes it ideal for use in chemical storage containers, laboratory equipment, and industrial piping systems. Unlike metals, it does not corrode, ensuring longevity in aggressive environments such as pharmaceutical manufacturing or wastewater treatment plants. -
Fatigue Resistance
Known for its ability to endure repeated bending or stress without cracking, polypropylene is widely used in applications requiring durability under cyclic loads. A notable example is its use in “living hinges” for bottle caps or flip-top containers, which can flex millions of times without failure. This property also benefits automotive components (e.g., battery casings) and consumer goods subjected to frequent use. -
Low Moisture Absorption
With a water absorption rate of less than 0.01% of its weight, polypropylene outperforms many plastics (e.g., nylon) in humid or wet environments. This minimal moisture uptake prevents swelling, warping, or electrical conductivity changes, making it suitable for marine applications, food packaging, and medical devices where hygiene and dimensional stability are critical. -
Electrical Insulation
Polypropylene’s high dielectric strength and low electrical conductivity make it a preferred material for insulating cables, capacitors, and circuit boards. Its non-conductive nature remains stable even in high-moisture conditions, ensuring safety in electrical housings, power tools, and telecommunications infrastructure. -
Cost-Effectiveness
As one of the most economical thermoplastics, polypropylene is cheaper to produce than alternatives like PVC or PTFE, owing to its low raw material costs and efficient melt-processing (e.g., injection molding, extrusion). Its recyclability further reduces lifecycle costs, driving adoption in packaging, textiles, and disposable products where budget constraints are paramount.
Disadvantages and Limitations
-
High Thermal Expansion
Polypropylene has a high coefficient of thermal expansion (≈150 µm/m·°C), causing significant dimensional changes under temperature fluctuations. This limits its use in precision engineering (e.g., aerospace components) or high-temperature environments unless paired with stabilizers or fillers like glass fiber to mitigate deformation. -
Susceptibility to UV Damage
Prolonged exposure to sunlight degrades polypropylene through photo-oxidation, leading to brittleness and discoloration. For outdoor applications (e.g., garden furniture, automotive bumpers), UV-resistant additives (e.g., carbon black, HALS stabilizers) or coatings are essential to extend service life. -
Poor Bonding Properties
The material’s non-polar surface resists adhesion, making it difficult to glue or paint without specialized surface treatments (e.g., plasma etching, flame treatment). Painting often requires primers, while bonding relies on epoxy or cyanoacrylate adhesives designed for polyolefins. -
Flammability
Polypropylene is highly flammable (ignition temperature ≈ 570°F/300°C) and melts rapidly when exposed to fire, releasing toxic fumes. In applications like electronics or construction, flame-retardant additives (e.g., brominated compounds, phosphorus-based agents) are necessary to meet safety standards (e.g., UL94).
Polypropylene vs. Other Materials
While polypropylene (PP) offers advantages such as chemical resistance, low density, and cost-effectiveness, it has distinct limitations compared to other polymers and traditional materials like metals, polyethylene (PE), or polyvinyl chloride (PVC). These drawbacks influence its suitability for specific applications and often necessitate modifications or compromises.
-
High Thermal Expansion
Polypropylene exhibits a high coefficient of linear thermal expansion (CLTE), typically around 100–150 μm/m·°C, which is 5–10 times greater than metals (e.g., aluminum: 23 μm/m·°C) and higher than engineering plastics like polyamide (nylon) or acrylonitrile butadiene styrene (ABS). This property causes dimensional instability under temperature fluctuations, leading to warping, stress cracking, or misalignment in tightly toleranced components (e.g., automotive parts, piping systems). In contrast, materials like glass-filled polymers or metals retain dimensional stability at elevated temperatures, making them preferable for high-precision or high-heat environments. -
Susceptibility to UV Degradation
PP lacks inherent UV resistance due to its hydrocarbon backbone, which undergoes chain scission and oxidation when exposed to sunlight. Over time, this results in surface embrittlement, discoloration, and loss of mechanical strength—issues less pronounced in UV-stable polymers like PVC, acrylics (PMMA), or polyethylene terephthalate (PET). To mitigate this, PP requires additives such as UV stabilizers (e.g., hindered amine light stabilizers, HALS) or carbon black (1–3% loading) for outdoor applications (e.g., garden furniture, automotive bumpers). Unmodified PP is unsuitable for long-term outdoor use compared to inherently weather-resistant materials like ASA (acrylonitrile styrene acrylate) or PTFE (Teflon). -
Poor Bonding and Surface Compatibility
PP’s non-polar, chemically inert surface and low surface energy (~30 mN/m) make adhesion challenging for paints, inks, and adhesives. Unlike polar polymers such as ABS or polycarbonate (PC), which bond readily with epoxy or cyanoacrylate adhesives, PP requires surface treatments (e.g., plasma, corona discharge, or flame treatment) to oxidize its surface and improve wettability. Specialty PP-specific adhesives (e.g., two-part acrylics) or primers are often needed, adding complexity and cost. In contrast, metals or treated plastics (e.g., primed PVC) offer superior adhesion for coatings and multi-material assemblies. -
Flammability
PP is highly flammable (UL94 HB rating), with a low limiting oxygen index (LOI ~17%), meaning it burns readily in air and drips molten material, posing fire risks. This contrasts sharply with inherently flame-retardant materials like PVC (LOI ~45–50%) or engineering thermoplastics like polyether ether ketone (PEEK, LOI ~35%). To meet safety standards in electronics, construction, or automotive applications, PP must be blended with halogenated or phosphorus-based flame retardants, nanoclays, or intumescent additives. However, these additives can compromise mechanical properties or recyclability—a trade-off unnecessary in materials like metal or ceramics, which are non-combustible.
Comparative Summary with Alternatives
-
Metals (e.g., steel, aluminum): Superior dimensional stability, UV resistance, and non-flammability but heavier and prone to corrosion.
-
PVC: Better UV and flame resistance but less flexible and contains chlorine, raising environmental concerns.
-
ABS: Easier to paint/bond and higher heat resistance but more expensive and less chemically resistant.
-
HDPE: Similar chemical resistance but lower melting point and greater susceptibility to stress cracking.
Design Implications
PP’s limitations necessitate careful material selection. For example, in outdoor applications, UV-stabilized PP competes with ASA or fiberglass-reinforced polymers, while in high-temperature environments, glass-filled PP or metals may be preferred. Its poor adhesion often drives the use of mechanical fasteners (e.g., screws, snap-fits) over adhesives. Despite these challenges, PP remains a top choice for disposable packaging, non-load-bearing components, and applications prioritizing lightweight, corrosion-resistant, and low-cost solutions—provided its drawbacks are addressed through additives or design adaptations.
Recycling Polypropylene (PP)
- Recycling Process: Involves melting, filtering contaminants, and reforming.
- Challenges: Sorting difficulties and degradation in mechanical properties.
- Uses of Recycled PP (rPP): Found in automotive parts, packaging, and textiles.
Health and Safety Concerns
Polypropylene is generally non-toxic and safe for food contact. However, burning PP releases harmful fumes, and prolonged UV exposure may degrade pp structure.
Additives to Improve Polypropylene Properties
Common additives include:
- UV stabilizers: Protect against sunlight degradation.
- Flame retardants: Reduce flammability.
- Impact modifiers: Enhance toughness.
- Antioxidants: Prevent thermal degradation.
Sustainability of Polypropylene
PP is recyclable and lightweight, reducing transportation emissions. However, challenges in sorting and degradation limit large-scale recycling. Bio-based PP is emerging as an eco-friendly alternative.
Polypropylene Suppliers and Brands
A major supplier is Shobeir Shimi, offering high-quality polypropylene products for industrial applications. Shobeir Shimi is an Iranian company specializing in the production and distribution of chemical products, including high-quality polypropylene for various industrial applications. Their offerings cater to sectors such as packaging, automotive, and textiles, providing materials known for durability and performance.
conclusion
Polypropylene is a highly versatile and widely used thermoplastic, valued for its durability, chemical resistance, and cost-effectiveness. Its applications span multiple industries, from packaging and automotive to textiles, medical devices, and agriculture. The material’s lightweight nature, recyclability, and adaptability make it a preferred choice for manufacturers seeking efficiency and performance. Despite some limitations, polypropylene remains essential in modern industrial and consumer products, demonstrating its significance in various sectors.

