Urea is perhaps the most important chemical compound in today’s industrial and agricultural scenario. It is the most concentrated Nitrogen fertilizer urea, used in the world, playing a vital role in food production worldwide, and in many industrial processes for emission reduction and efficiency enhancement. Chemically, urea is known as carbamide or the diamide of carbonic acid and exists as a simple, yet versatile, organic molecule represented by CH₄N₂O. The main uses of urea are as a major nitrogen source in agriculture and also as a feedstock or reactant in various technical and industrial applications.

What is Urea? A Chemical and Physical Profile
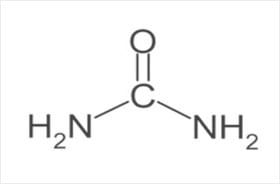
The Chemical Identity of Urea (CH₄N₂O)
The chemical structure of urea is the diamide of carbonic acid, with the molecular formula being CH₄N₂O, consisting of two amine groups (-NH₂) connected to a carbonyl group (C=O). UREA is, by nature, the end product of metabolic nitrogen excretion in mammals, though it is synthesized from inorganic sources in commercial forms. It is a highly stable compound, non-volatile at room conditions, and remarkably soluble in water, making it very suitable for agricultural and chemical urea uses.
Key Physical Properties & Specifications
The technical properties of urea have a direct impact on its performance in both urea fertilizer uses and industrial Urea Applications. The high Urea nitrogen content, usually around 46%, represents the most valuable property and makes it the most concentrated solid nitrogen source commercially available. The grade and quality are further defined by other parameters like biuret level, moisture content, and particle uniformity.
| Parameter | Typical Specification | Relevance |
|---|---|---|
| Chemical Formula | CH₄N₂O | Identifies urea as the diamide of carbonic acid |
| Nitrogen Content | 46% (Urea 46% Nitrogen Content) | Determines fertilizer efficiency |
| Melting Point | 132–135 °C | Affects processing and storage stability |
| Solubility in Water | 1080 g/L at 20 °C | Influences dissolution and application rate |
| Biuret Content | < 1.0 % (fertilizer grade) | Important for foliar safety and crop compatibility |
| Moisture Content | < 0.5 % | Prevents caking and ensures flowability |
Urea Granular vs. Prill vs. Powder: Understanding the Forms
Differences in particle size and manufacturing methods account for the inconsistencies in Urea granules, Urea powder, and Urea prills:
Urea Granular vs. Prill vs. Powder: Understanding the Forms
From Ammonia and CO₂: The Haber-Bosch and Urea Synthesis
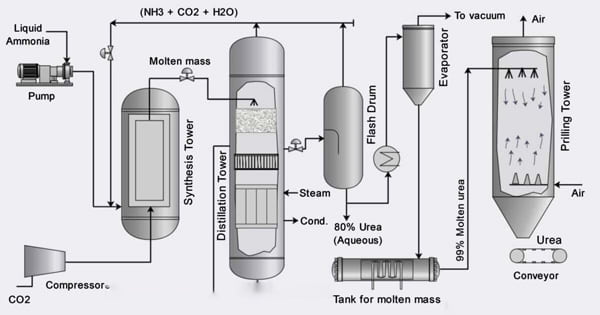
Industrial production of urea incorporates two major processes. First, the Haber-Bosch process synthesizes ammonia from nitrogen and hydrogen: Second, ammonia is reacted with carbon dioxide – also generated as a byproduct of ammonia production – at high pressure (140-250 bar) and temperature (170-200 °C) to form ammonium carbamate, which is then dehydrated to yield urea and water:
2NH3 + CO2 → NH2COONH4 → CH4N2O + H2O
Controlling Quality: Biuret, Moisture, and Free Ammonia
During synthesis, excess temperature or residence time creates biuret, which is an impurity formed by urea condensation. High levels of biuret (> 1%) are undesirable in agricultural and pharmaceutical applications due to their phytotoxicity. The function of urea quality control, hence, includes:
- Biuret concentration monitoring for fertilizer and feed safety.
- Reducing moisture content to prevent caking and degradation.
- Minimizing free ammonia to improve product stability and reduce corrosiveness.
How is Urea Made? The Industrial Manufacturing Process
From Ammonia and CO₂: The Haber-Bosch and Urea Synthesis
Industrial production of urea incorporates two major processes. First, the Haber-Bosch process synthesizes ammonia from nitrogen and hydrogen: Second, ammonia is reacted with carbon dioxide – also generated as a byproduct of ammonia production – at high pressure (140-250 bar) and temperature (170-200 °C) to form ammonium carbamate, which is then dehydrated to yield urea and water:
2NH3 + CO2 → NH2COONH4 → CH4N2O + H2O
Controlling Quality: Biuret, Moisture, and Free Ammonia
During synthesis, excess temperature or residence time creates biuret, which is an impurity formed by urea condensation. High levels of biuret (> 1%) are undesirable in agricultural and pharmaceutical applications due to their phytotoxicity. The function of urea quality control, hence, includes:
- Biuret concentration monitoring for fertilizer and feed safety.
- Reducing moisture content to prevent caking and degradation.
- Minimizing free ammonia to improve product stability and reduce corrosiveness.
A Detailed Breakdown: Grades of Urea and Their Standards
Fertilizer Grade Urea (Urea 46% Nitrogen)
One of the most frequently asked questions is: what is urea fertilizer, what grades does it have, and what are the urea fertilizer benefits? Fertilizer-grade Urea 46% nitrogen content, is one of the most widely used, efficient nitrogen sources in plants. It supports chlorophyll and protein production, can be applied to soil or in solutions, and is non-toxic, non-corrosive, and in harmony with precision farming.
| Grade | Purity (wt%) | Nitrogen Content (wt%) | Biuret (max wt%) | Moisture (max wt%) | Typical Use |
|---|---|---|---|---|---|
| Fertilizer Grade UREA | 99 | 46 | 1 | 0.5 | Agricultural nitrogen fertilizer |
| Technical Grade UREA | 99.5 | 46.3 | 0.3 | 0.3 | Chemical and resin manufacturing |
| DEF/AdBlue Grade | ≥ 99.7 | 46.6 | 0.2 | 0.2 | Diesel exhaust treatment |
| Feed Grade UREA | ≥ 99.0 | 46 | 0.5 | 0.5 | Ruminant feed supplement |
| Pharma/Cosmetic Grade | ≥ 99.8 | 46.5 | 0.1 | 0.1 | Medical, dermatological use |
Technical Grade Urea
Technical urea is 99-99.5% pure with low biuret and moisture; most of it goes into fast-growing urea-formaldehyde and melamine-formaldehyde resins, adhesives, and fine chemical syntheses, in prill or powder form, depending on the type of use of urea.
DEF Grade Urea (AdBlue) & The ISO 22241 Standard
DEF (AdBlue) urea is a highly purified Industrial urea that meets ISO 22241, limiting contaminants to protect SCR catalysts. Dissolved in deionized water at 32.5%, it is used to convert NOₓ emissions from diesel into nitrogen and water.
Feed Grade Urea
Feed-grade urea provides non-protein nitrogen to ruminants. Microbes convert it into ammonia for protein synthesis, which aids growth when the application is balanced with energy. Low biuret and impurities prevent toxicity.
Pharmaceutical & Cosmetic Grade Urea
This grade is of the highest purity, with the most stringent Urea Specifications. This grade of urea used for pharmaceutical formulations, dermatological creams-as a keratolytic and moisturizer cosmetic emulsions. The function of urea in this case is to enhance skin hydration and improve active ingredient absorption. Due to its biocompatibility and mildness, it finds wide application in creams with a 5-10% urea concentration.
The Versatile Applications of Urea Across Industries
Agriculture: The World’s Leading Nitrogen Fertilizer
UREA is an effective fertilizer, relatively easy to handle, and compatible with other fertilizers. Urea applied in agriculture provides the much-needed nitrogen for photosynthesis, growth, and yield. It is also an ingredient in the production of other fertilizer urea, such as urea-ammonium nitrate (UAN) and controlled-release products. Is urea organic? While it contains carbon, synthetic urea is, by definition, inorganic since it does not have a biological origin.

Automotive: Powering Clean Diesel with AdBlue & DEF
The purity of urea solutions for agriculture and transport differs, but the chemistry is basically the same. In SCR systems, Industrial urea solution (32.5%) reduces nitrogen oxides (NOₓ) emissions by converting them into N₂ and H₂O. This technology is under the mandate of Euro VI and EPA standards; hence, DEF-grade urea becomes indispensable in sustainable transport.
Manufacturing: The Backbone of Resins and Adhesives
Technical Grade Urea is a significant reactant in the fabrication of urea-formaldehyde and urea-melamine-formaldehyde resins. These polymers find their applications in MDF, plywood, and particleboard, along with coatings and adhesives. The highly reactive nature of Chemical urea gives rise to precise polymer cross-linking and its thermally stable characteristics in final composites.
Chemical Synthesis, Pharma, and Cosmetics
Apart from fertilizers, the Industrial grade urea also finds applications as an important intermediate in the synthesis of melamine, cyanuric acid, and dyes. In pharmaceuticals, it acts as a stabilizer and excipient. In cosmetics, topical application of urea enhances dermal hydration and barrier repair.
Niche Applications: From De-Icing to Animal Feed
Industrial urea is used in de-icing formulations because it is a less corrosive alternative to chloride salts. It’s also used as a nitrogen source in animal feed, in textile dyeing, and in food processing as a dough conditioner or yeast nutrient.
The Commercial Landscape: How to Buy & Source Urea
Navigating the Market: Suppliers, Manufacturers, and Distributors
There are integrated ammonia-Urea manufacturers, while distributors handle packaging, transportation, and trading. They specialize in different grades; the product must be traceable, and shipment requirements must be met.
Procurement Guide: Bulk Orders, Pricing, and Logistics
During the planning of a Urea bulk order, determinants of price include global ammonia supply, natural gas costs, seasonal agricultural demand, and freight rates. The regulations regarding Urea import and Urea export also vary from region to region. Many of them require certificates of analysis, CoA, and adhere to either REACH or ISO standards. Shobeir Shimi, for one, is a company specializing in commercial and agricultural supply, including urea fertilizer, and Technical grade urea with full logistics support for bulk orders and containerized exports.
Finding the Right Partner: Sourcing by Grade
The buyer should select a Urea supplier based on consistency, purity documentation, and grade-specific standards. For sourcing, request Urea Specifications and CoAs to ensure it will work for your application-agriculture, industrial, or automotive. A reliable Urea distributor provides specific grades with warranted purity, traceability, and prompt logistics.
Safe Handling and Storage of Urea
Best Practices for Storing Urea to Maintain Quality
Since urea is hygroscopic, it should be stored in a cool, dry, and ventilated place away from water, moisture, and incompatible substances. Correct storage avoids caking and degradation and maintains a 46% nitrogen content to achieve all the specifications. For bulk storage and export, the silos and packaging shall be moisture-proof.
Urea Handling Precautions and Safety
Standard hygiene practices must be observed when handling urea, especially in dust form. Urea use of protective masks, gloves, and goggles prevents inhalation, skin contact, or eye irritation. Work areas should be well-ventilated to avoid skin and eye irritation. For full safety details, refer to the product’s SDS. Proper containment and the use of PPE ensure safe handling of urea during importation or exportation, or at distribution services.
Conclusion
In answer to the question “What is urea used for?”, we can say that urea has very important applications in agriculture and industry, ranging from its use as a nitrogen source in the use of urea fertilizer to its role as a major reactant in polymers and resins, as well as a clean-air additive in diesel exhaust systems. The basis for the versatility of this fertilizer is its specifications and tailored grades, such as Fertilizer, Technical, and Industrial urea. Proper knowledge of urea grades and storage can ensure quality and efficiency.
Contact us today for expert advice on sourcing high-purity technical or DEF-grade urea.
Urea: Frequently Asked Questions (FAQs)
- What is the main difference between technical grade and fertilizer grade urea?
Technical grade urea has higher purity, lower moisture, and lower biuret content and is used industrially. The urea fertilizer grade is less pure and contains higher biuret, used in agriculture.
- What is biuret in urea, and why is it important to control?
Biuret is an impurity formed during the process of heating urea and is toxic to some plants; it is also unsuitable for chemical synthesis, so low-biuret urea is required.
- How does urea in AdBlue/DEF reduce diesel emissions?
In the SCR system, urea is turned into ammonia, which reacts with NOx in the exhaust gases to form nitrogen and water.
- Is urea a natural or synthetic compound?
Urea occurs naturally in living organisms, but Industrial urea is prepared synthetically from ammonia and carbon dioxide.
- What are the most important factors when storing bulk urea?
Keep it dry; avoid moisture, heat, and incompatible chemicals such as nitrates.
