What is Polyethylene Terephthalate (PET)? Polyethylene Terephthalate, otherwise referred to by its chemical generic name PET or PETE, is a thermoplastic resin polymer that is a polyester. It is primarily recognized for its suitability to be utilized in broad applications and is used extensively in the packaging, textiles, and consumer goods sectors. The pet structure is favorable for the manufacture of lightweight yet strong materials and is, therefore, a common material when making bottles for beverages, food packaging, and man-made fibers.
PET’s significance in daily life is due to its unique set of properties. PET possesses excellent impact resistance, humidity, alcohol, and various solvents resistance, which ensures product stability under multiple conditions. It is also readily recyclable, aligned with modern sustainability requirements. PET recyclability has led to extensive usage and introduced successful recycling streams globally.
PET grades
| GRADE | PRODUCER | MFR | DENSITY | DATASHEET |
|---|---|---|---|---|
| BG825 | TONDGOYAN | — | — | download |
| BG821 | TONDGOYAN | — | — | download |
| BG732 | TONDGOYAN | — | — | download |
| BG785 | TONDGOYAN | — | — | download |
| BG781 | TONDGOYAN | — | — | download |
Chemical Structure and Composition of pet
The pet chemical structure is characterized by repeating units with the polymerization of two primary monomers: Monoethylene glycol (MEG) and terephthalic acid (PTA). The Polyethylene terephthalate structure includes ester linkages that are obtained through polycondensation, forming a linear polymer backbone with aromatic rings, with the contribution of terephthalic acid units. The unique pet composition is accountable for the mechanical strength and heat stability of the polymer. The general chemical formula for PET is (C10H8O4)n, representing the repeating esterified units in its structure. The polyethylene terephthalate structural formula has a backbone of alternating units between ethylene glycol and aromatic terephthalate groups. The molecular structure determines the structure of pet and its properties.
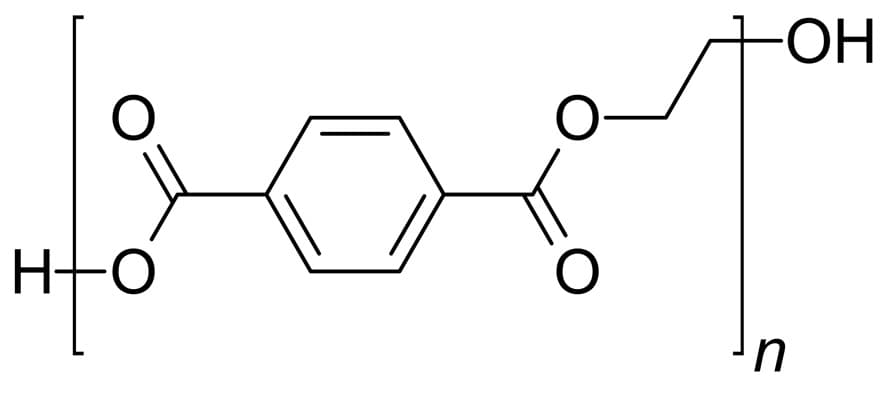
In production, Polyethylene Terephthalate is manufactured via two primary paths: the Dimethyl Terephthalate (DMT) path and the Terephthalic Acid (PTA) path. Both involve polycondensation reactions within catalysts and additives that ensure high polymerization yields and end-product properties. Recent advances have just made available Bio-PET, a green alternative where some petrochemical-derived monomers are substituted by renewable sources with a lower carbon footprint than traditional PET production.
Key Properties of PET that Aid in Material Selection
Polyethylene terephthalate, or PETE, also polyethylene terephthalate polymer, is simply well known for its diverse material properties that have made it a selection material for a range of industrial applications. It is imperative to research polyethylene terephthalate properties as part of the material selection for industries ranging from packaging to engineering parts.
Physical Properties
Polyethylene Terephthalate possesses excellent flexibility, colorability, and transparency, which are critical for applications such as clear packaging. PET’s tensile strength is extremely high, providing good mechanical performance at low weight. This combination of strength and stiffness at low density leads to very good dimensional stability over a variety of environmental conditions. In addition, PET’s shatter-resistance allows it to find application as a safer alternative to glass in containers for food and liquids. PET’s intrinsic viscosity and molecular weight indicate the polymer chain length and have a direct influence on mechanical strength and processability.
Barrier Properties
Another major attribute of the polyethylene terephthalate polymer is its excellent barrier properties. PET has low gas permeability, thus limiting the passage of oxygen and carbon dioxide effectively, which is necessary in a bid to preserve the freshness of foodstuffs. Additionally, PET resistance to moisture intrusion helps avoid degradation of product integrity over time.
Thermal Properties
Polyethylene Terephthalate’s thermal properties enable it to be employed at a broad range of temperatures from -60°C to 130°C. Its glass transition temperature (Tg), one of the fundamental properties established by the pet chemical structure, is usually at about 70-80°C, where it transforms from a glassy to a rubbery condition. It also has distinctive melting (Tm) and crystallization behavior, with crystallization kinetics influencing its final mechanical and optical properties. Heat distortion temperature (HDT) also refers to PET’s load stability at elevated temperatures.
Electrical Properties
Polyethylene Terephthalate is an excellent electrical insulator, having high dielectric strength, making it suitable for electrical and electronic applications requiring good insulation.
Chemical Properties
The chemical resistance of Polyethylene Terephthalate is good for many environments. It is resistant to oils, greases, alcohols, and dilute acids. It has moderate resistance to alkaline chemicals and some hydrocarbons. This chemical stability is an important factor in PET finding application in medical devices and food packaging.
Food Contact Safety
Since polyethylene terephthalate (pet or pete) is an inert and non-reactive material, it has been recognized for direct food contact by the FDA as well as other regulatory bodies, and it is found to be safe for packaging.
Microwave Transparency and Flavor Absorption
Polyethylene Terephthalate is transparent to microwaves, allowing safe food heating in packages without affecting the integrity of the package. It also absorbs virtually no flavor, maintaining the natural taste and aroma of packaged products.
Manufacturing Process of PET
In answer to the question “How is polyethylene terephthalate made?” it must be said that this is made through a chemical process called PET polymerization. The main ingredients include ethylene glycol (EG), terephthalic acid (PTA), and dimethyl terephthalate (DMT). So, these things react together. The ethylene glycol bits hook up with the terephthalic acid or dimethyl terephthalate bits. When this happens, water or methanol is released. The result is long chains of PET polymer.

Following polymerization, the Polyethylene Terephthalate melt is extruded and fed through a die to form continuous strands. The strands are quenched rapidly to lock in the polymer and subsequently pelletized into small-sized granules. The pellets are intermediate intermediates for other processing into fibers, films, or molded products. Proper control of temperature and reaction conditions during polymerization and extrusion delivers the desired mechanical and thermal properties of the resultant PET product.
Limitations of Polyethylene Terephthalate (PET)
Despite owning its valuable properties, Polyethylene Terephthalate is prone to the limitations inherent in it that influence its performance in certain uses.
Mechanical and Moldability Constraints
Polyethylene Terephthalate that has become crystalline will possess reduced impact strength as well as reduced moldability. This limitation constrains its use in applications involving high toughness or complex mold shapes in its crystalline state.
Chemical Vulnerabilities
Amorphous Polyethylene Terephthalate is most susceptible to degradation in conditions of boiling water, alkaline solutions, and strong bases. Hydrolytic degradation may occur under these conditions at the cost of mechanical strength.
Susceptibility to Certain Chemicals
Polyethylene Terephthalate chemical resistance is compromised by exposure to ketones, chlorinated and aromatic hydrocarbons, and diluted bases or acids at elevated temperatures. These chemicals can cause scission of the polymer chains or swelling of the polymer, degrading pet properties.
Thermal Degradation
PET can be thermally degraded with poor temperature control during processing and causing reduced molecular weight and tensile strength of pet. Thermal sensitivity demands strict process conditions under polyethylene terephthalate polymerization and molding.
Acetaldehyde Formation
One of the major PET limitations, particularly in the packaging of food and beverages, is acetaldehyde formation as a result of thermal processing. Acetaldehyde is a volatile compound with off-odors and tastes that can have a detrimental effect on product quality.
Types and Modifications of PET
Petroleum for polymerization has numerous alterations designed for specific industrial requirements. An example of this is Polyethylene Terephthalate Glycol-modified (PETG). It has modifiers like cyclohexane dimethanol (CHDM) and isophthalic acid, which are added during polymerization. They lower the polymer chain order, thus lowering crystallinity and improving processability, impact resistance, and clarity, which makes thermoforming easier. Therefore, PETG is used where high optical properties are required. In addition to PETG, Co-PET represents the copolymers of PET synthesized by adding other monomers, which change the crystallization of the polymer and its mechanical properties. Such copolymers are designed to a particular specification to cater for increasingly varied applications of polyethylene terephthalate.
Besides, Polyethylene Terephthalate is frequently blended with other polymers, such as thermoplastics, thermosets, and rubbers, to maximize performance properties and affordability. Some of the popular blends include PET/polycarbonate (PET/PC), a compromise of toughness and enhanced chemical resistance, PET/polybutylene terephthalate (PET/PBT), with high impact strength and improved crystallization rates, and PET/polyolefin blends, which are designed to achieve a trade-off between mechanical properties and reduced material prices. These modifications expand the application window of PET, demonstrating the versatility of the polymer in various fields.
Key Applications of Polyethylene Terephthalate
Polyethylene terephthalate (PET) is a general-purpose thermoplastic polymer used extensively in a wide range of industries due to its valuable chemical structure that possesses excellent mechanical and thermal properties. Its pet composition, primarily consisting of repeating ester units of ethylene glycol and terephthalic acid, possesses a balance of durability, strength, and chemical resistance.
Packaging
The most apparent application of polyethylene terephthalate is in packaging, in the form of food and drink containers. Polyethylene terephthalate examples are trays, jars, bottles, and blister packs. Its light weight, clarity, and good barrier properties are the best way to guarantee product freshness and appearance. Films also find extensive application in flexible packaging with good mechanical strength and barrier to water at a reasonable cost, polyethylene terephthalate.

Textile Industry
Polyethylene Terephthalate is the basis of polyester fibers, which are applied extensively in clothing, carpets, fleece jackets, and comforter fill. The chemical stability and strength of PET due to its tensile strength make the fabrics wear and tear resistant. PET is also applied to mesh fabrics, filters, and bracing wires in industrial applications, where chemical stability and strength are of critical concern.

Automotive Industry
Its components, such as wipers, headlamp retainers, engine covers, and others, capitalize on the high mechanical strength and wear and weather resistance of PET because of its pet glass transition temperature, which allows the material to operate well in a wide range of temperatures.

Electrical and Electronics
Due to its excellent electrical insulation characteristics and chemical resistance, PET finds application in insulating components, smart meters, and solar junction boxes. Its electrical stress resistance makes it suitable for these rigorous applications.

Other Specialized Applications
I’s variety also manifests in specialized Polyethylene terephthalate uses such as photovoltaic panels, submarine cables, manufacturing nanodiamonds, and even consumer products like glitter and water paper. All these applications highlight PET’s adaptability based on its unique polymer composition and physical characteristics.

PET in Comparison to Other Common Plastics
Polyethylene terephthalate (PET) is often compared to other plastics due to its specific set of properties, which will render it desirable or limited with respect to alternatives.
- PET vs. PBT (Polybutylene Terephthalate): Both polyesters; they differ as PET tends to have greater tensile strength of pet and greater rigidity, whereas PBT offers greater flexibility and enhanced chemical resistance. PET’s marginally higher glass transition temperature provides greater heat performance in certain applications.
- PET vs. HDPE (High-Density Polyethylene): PET has greater clarity and barrier properties than HDPE, but is more likely to be more stress-crack sensitive under certain conditions. HDPE is stronger against impact and heat withstanding but PET’s appearance and recyclability make it more popular for packaging.
- PET vs. PVC (Polyvinyl Chloride): PET is more recyclable and less susceptible to UV degradation than PVC. While PVC can be less expensive, its enhanced durability and green image make it more viable for packaging food and beverages.
- PET vs. Polycarbonate (PC): Polycarbonate yields higher impact resistance and mechanical strength, which is well-suited to more demanding structural applications. PET, however, is better chemically resistant and less stress-cracking prone, which makes it better suited to applications requiring long-term integrity and transparency.
- PET vs. Acrylic: Acrylic possesses more impact strength along with better UV stability, but not the barrier characteristics or recyclability of PET. PET’s affordability and versatility lead to acrylic’s advantages being displaced in packaging and textiles.
- PET vs. BOPP (Biaxially Oriented Polypropylene): PET is stiffer and clearer, and it has better barrier properties against moisture and gases compared to BOPP. BOPP is more flexible but is used where moisture barrier and cost are the priority considerations.
Recycling of Polyethylene Terephthalate (rPET)
Polyethylene Terephthalate or PETE, best known by its resin code #1, is one of the most recyclable plastics due to the desirable properties and rather low lifetime used in consumer products. Impact resistance, clarity, and lightness make the product a favorite for bottles employed in beverages and packaging purposes, but the same attributes also enable efficient collection and recycling. PET’s recyclability is also propelled by infrastructure to establish sorting and the potential of being converted into a broad array of secondary products, which supports circular economy principles.
Polyethylene Terephthalate recycling is a process in several steps, beginning with washing to be impurity-free, and then chemical treatments in order to depolymerize the polymer chains if needed. The most prevalent process, mechanical recycling, involves shredding and remanufacturing of PET flakes into new pellets and is chosen for its low Polyethylene terephthalate price and lightness on the environment. Chemical recycling processes of hydrolysis, methanolysis, glycolysis, and enzymatic depolymerization enable recovery of monomers to yield engineering-grade rPET with properties close to virgin material. The recycled PET (rPET) thus obtained is used for different applications, from textile fibers to food-grade containers, car parts, films, sheets, and strapping material.
In addition to expanding the Polyethylene Terephthalate lifecycle, these applications bring significant environmental benefits in the form of reducing energy consumption and greenhouse gas emissions associated with the production of virgin PET. Industry initiatives continue to drive PET recycling through improved collection networks and facilitating policies, while research into bacterial and enzymatic biodegradation continues to offer promising avenues to further reduce plastic pollution.
Safety and Environmental Considerations
PET (polyethylene terephthalate common name known) is deemed biologically inert and generally harmless to contact and oral ingestion, the foundation upon which its widespread use as a food and beverage package material has come about. However, chemical leaching issues remain present, primarily the migration of antimony, a catalyst residue used in Polyethylene Terephthalate production, from packaging into foods. While normally low in concentration, such migration necessitates regulatory monitoring due to potential endocrine-disrupting activity upon long-term exposure. In landfill conditions, PET’s chemical inertness is an aspect of low chemical toxicity and space economy compared to biodegradable materials, but its durability is an issue for long-term environmental accumulation.
Moreover, microfibres emitted by Polyethylene Terephthalate fabrics are a new environmental problem that disperses widely in aquatic ecosystems and may affect marine species. Resolution of such safety and environmental concerns remains a matter of concern for the sustainable application of PET as well as waste management practices.
Conclusion
Polyethylene Terephthalate (PET) is a cornerstone of modern material science, recognized for its exceptional strength, clarity, and widespread recyclability, making it indispensable across packaging, textiles, and automotive industries. Its unique chemical structure, formed from ethylene glycol and terephthalic acid, provides superior mechanical properties, heat stability, and barrier characteristics that preserve product integrity. Despite minor limitations like reduced impact strength in crystalline forms and susceptibility to certain chemicals, ongoing advancements such as Bio-PET and various modifications like PETG continually broaden its applications and address these challenges.
PET is one of the most recycled plastics globally, with both mechanical and chemical recycling methods transforming it into new products, significantly reducing its environmental footprint. While generally considered safe and biologically inert, continuous monitoring of potential chemical leaching and the impact of microfibers remains essential for its sustainable use. Ultimately, PET’s enduring versatility and the persistent efforts to enhance its properties and recyclability underscore its critical role in shaping a more sustainable future for material consumption and production.

